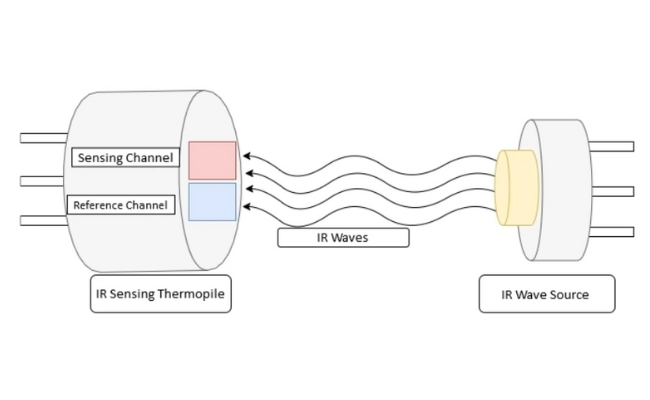
Working Principle of infrared gas detector
The working principle of a gas detector depends on the type of sensor technology it uses. Here's a general overview:
Catalytic Bead Sensors: These sensors consist of two coils of fine platinum wire coated with a catalyst. One coil is electrically heated to a high temperature, while the other is used as a reference. When combustible gas comes into contact with the hot coil, it oxidizes, causing a change in resistance that is proportional to the gas concentration.
Infrared (IR) Sensors: IR sensors measure infrared light absorption by gases at specific wavelengths. A source emits infrared light through the gas sample, and a detector measures the intensity of light that reaches it. The amount of light absorbed is proportional to the gas concentration.
Electrochemical Sensors: These sensors use a chemical reaction to detect gases. When the target gas comes into contact with the sensor's electrodes, it causes a chemical reaction that generates an electrical signal. The magnitude of the signal is proportional to the gas concentration.
Photoionization Detectors (PID): PID detectors use ultraviolet (UV) light to ionize gas molecules, creating positively charged ions. These ions are then collected on electrodes, generating a current proportional to the gas concentration.
Metal Oxide Semiconductor (MOS) Sensors: MOS sensors consist of a thin film of metal oxide that changes electrical conductivity when exposed to a target gas. The change in conductivity is measured and used to determine the gas concentration.
Regardless of the sensor technology, gas detectors typically include a display or alarm system to indicate the presence of gas above a certain concentration. They may also include features such as data logging, wireless connectivity, and self-testing capabilities for improved safety and reliability.